Current Research Projects:
I. Energy Storage
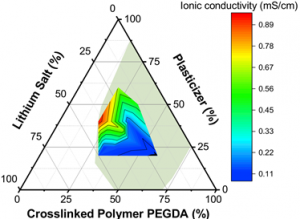
Solid-state battery. Lighter and more durable batteries will increase the range of Electric Vehicles and lower the total battery cost per vehicle. Lithium metal is the best potential anode material due to its large capacity and low charging voltage. However, traditional liquid electrolytes can neither prevent lithium dendrite formation nor are stable with respect to the lithium metal during prolonged cycles. To realize the potential advantages of a lithium metal anode and achieve safe operation, all-solid-state lithium metal batteries (ASSLMBs) have been of great interest. The solid-state electrolyte is the key component for ASSLMBs. Our research group is currently dedicated to the development of materials used for solid-state electrolyte.
See our recent publication of superionic conductive solid polymer electrolyte:
- Si Li, Yu-Ming Chen, Wenfeng Liang, Yunfan Shao, Kewei Liu, Zhorro Nikolov and Yu Zhu A Superionic Conductive, Electrochemically Stable Dual-Salt Polymer Electrolyte. Joule 2 (9), 1838-1856, 2018. https://www.cell.com/joule/fulltext/S2542-4351(18)30244-7
- Wenfeng Liang, Yunfan Shao, Yu-Ming Chen, and Yu Zhu A 4 V Cathode Compatible, Superionic Conductive Solid Polymer Electrolyte for Solid Lithium Metal Batteries with Long Cycle Life. ACS Appl. Energy Mater., 2018, 1 (11), 6064–6071. https://pubs.acs.org/doi/pdf/10.1021/acsaem.8b01138
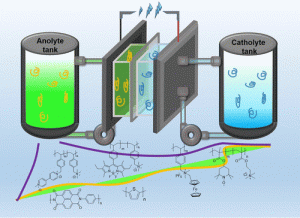
Redox Flow battery. Redox flow battery (RFB) is one of the most promising systems for large scale electrochemical energy storage applications. The development of redox-active materials is an essential part for RFB research. Commercial RFBs utilize redox-active inorganic ions, which have several issues such as expensive and toxic active materials, crossover of redox species and high cost of ion exchange membrane (IEM). The incorporation of redox-active organic materials is an intriguing solution because low-cost organic redox-active materials and size-exclusion porous membranes formed by commodity polymers could be applied to replace expensive inorganic redox-active materials and ion exchange membranes, respectively. Our research group focuses on the development of aqueous organic redox-active materials in RFB application.
See our recent publication of redox flow batteries:
- Yun-Yu Lai, Xiang Li and Yu Zhu Polymeric Active Materials for Redox Flow Battery Application. ACS Appl. Polym. Mater. 2020, 2, 2, 113-128. https://pubs.acs.org/doi/abs/10.1021/acsapm.9b00864?fbclid=IwAR1XfrYlsnKVcbKX76uFiOSZHTHY232ZeCoVA_j2-RiN6ooY0YqemL9pQHs
- Yun-Yu Lai, Xiang Li, Kewei Liu, Wei-Yao Tung, Chung-Fu Cheng and Yu Zhu Stable Low-Cost Organic Dye Anolyte for Aqueous Organic Redox Flow Battery. ACS Appl. Energy Mater. 2020, 3, 2290–2295. https://pubs.acs.org/doi/pdf/10.1021/acsaem.9b01735
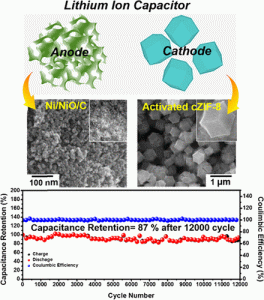
Nanomaterials for Energy Storage. Low-dimensional materials, such as graphene and carbon nanotubes (CNT) exhibit exceptional high carrier mobility, high electrical and thermal conductivity and large specific surface area. By controlling synthesis and assembly, those exotic properties are able to be extended to macro-scale, which is important for applications like solar cells, supercapacitors and lithium ion batteries. Currently we are exploring the techniques to synthesis and assembly well-organized 3D bi-continuous low-dimensional materials. Those materials are kinetically ideal electrodes/supports for electrochemical energy storage devices, which is important for electrode’s reaction mechanism and performance studies.
Some recent reports from our group:
- Chung-Fu Cheng, Xiang Li, Kewei Liu, Feng Zou, Wei-Yao Tung, Yi-Fan Huang, Xuhui Xiac, Chien-Lung Wang, Bryan D.Vogt and Yu Zhu A high-performance lithium-ion capacitor with carbonized NiCo2O4 anode and vertically-aligned carbon nanoflakes cathode. Energy Storage Materials, 2019, 22, 265–274266. https://www.sciencedirect.com/science/article/pii/S2405829719308943
- Feng Zou, Yu-Ming Chen, Kewei Liu, Zitian Yu, Wenfeng Liang, Min Gao, Sarang M. Bhaway and Yu Zhu Metal Organic Frameworks Derived Hierarchical Hollow NiO/Ni/Graphene Composites for Lithium and Sodium Storage. ACS Nano, 2016, 10 (1), 377–386. http://pubs.acs.org/doi/abs/10.1021/acsnano.5b05041
Advanced materials for battery manufacturing. Advanced manufacturing technology are crucial for low-cost energy storage devices. 3-D printing techniques could be a highly automated manufacturing technique, which would enable the advanced battery technology to be scaled at low manufacturing cost. In addition, mask-less 3D-printing deposition methods are of particular interest to provide intimate contact between the electrode and electrolyte layers, improving battery performance. Our research group are developing new materials that will address the challenges during the manufacturing of advanced energy storage devices. The current research topics includes polymer binder materials for silicon anode, printable electrolyte/electrode for additive manufacturing.
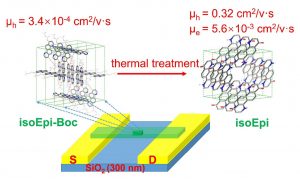
II. Organic Electronics
Hydrogen bonded conjugated materials. Hydrogen-bonding is a strong and directional intermolecular interaction. It has potential to be used to “lock in” molecular packing and improve the long-range order of organic molecules. Our group is dedicated to develop the conjugated polymers with hydrogen-bonding on the main chain, which is targeted to form desired molecular packing between conjugated polymer molecules. Furthermore, the dynamic formation of hydrogen-bonding can provide additional functions such as patentability to the polymers.
See our recent publication:
- Haichang Zhang, Wei-Yao Tung, Xiang Li, Hailiang Jin, Ruonan Deng, Yu-Ming Chen, Yifan Mao and Yu Zhu Conjugated polymer with dynamic and thermoreversible hydrogen bonding on the backbone. Polymer, 203 (2020), 122787. https://doi.org/10.1016/j.polymer.2020.122787
- Haichang Zhang, Ruonan Deng, Jing Wang, Xiang Li, Yu-Ming Chen, Kewei Liu, Clinton J. Taubert, Stephen Z. D. Cheng, and Yu Zhu Crystalline Organic Pigment-Based Field-Effect Transistors. ACS Appl. Mater. Interfaces 2017, 9, 21891−21899. http://pubs.acs.org/doi/abs/10.1021/acsami.7b03170
III. Polymer composites with 2D fillers
Two-dimensional (2D) nanomaterials, such as graphene, have ultrahigh Young’s modulus and ultimate strength. They are ideal candidates to improve and extend properties of polymers. In addition, 2D sheets are expected to achieve percolation in polymer composites at ultralow concentrations. Our group are currently working on composites with low dimensional fillers for improving the conductivity.